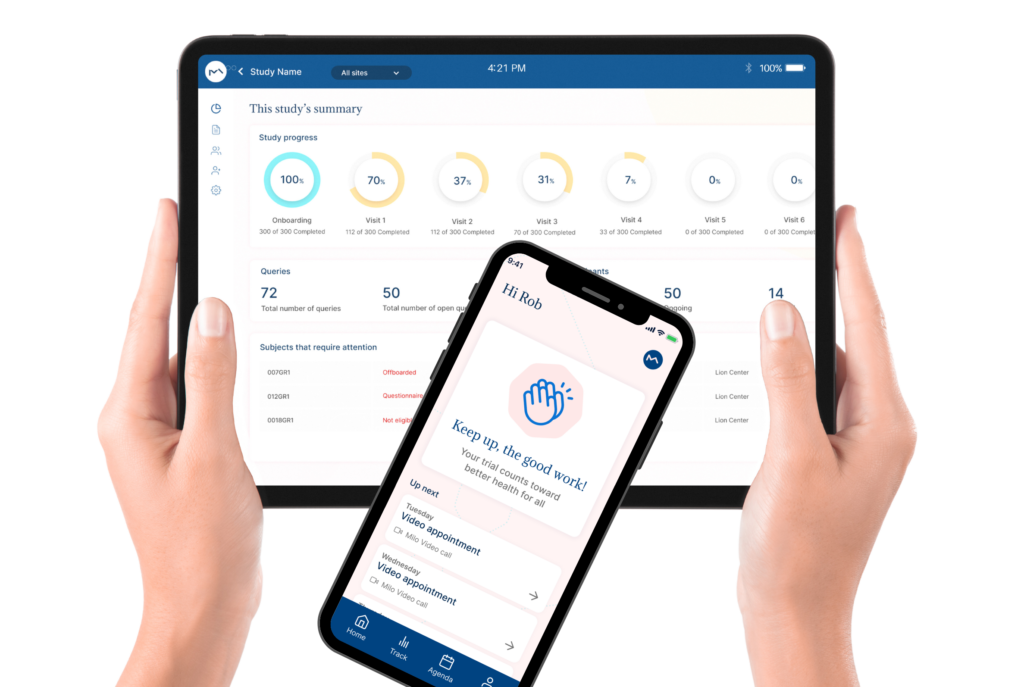
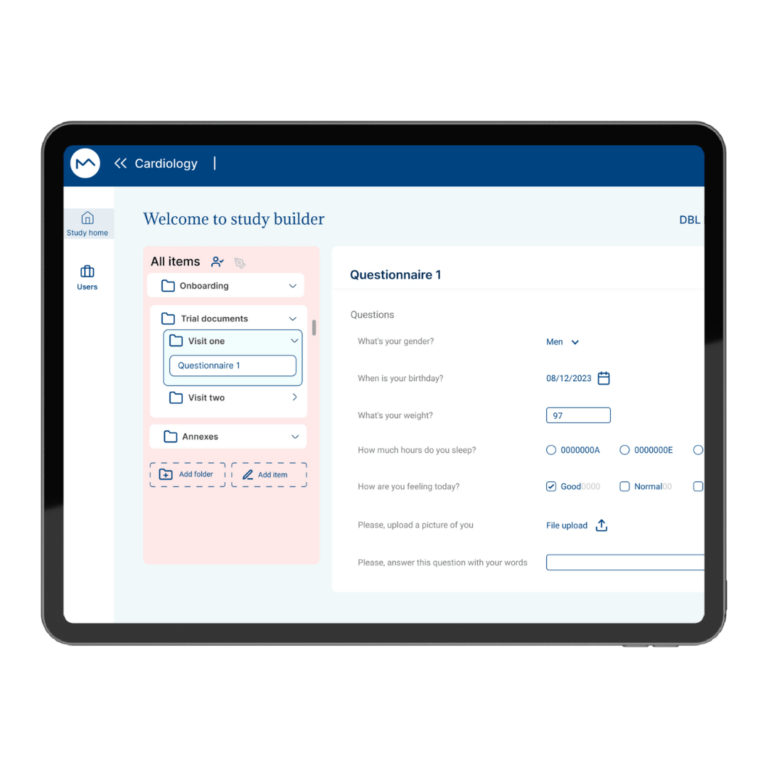
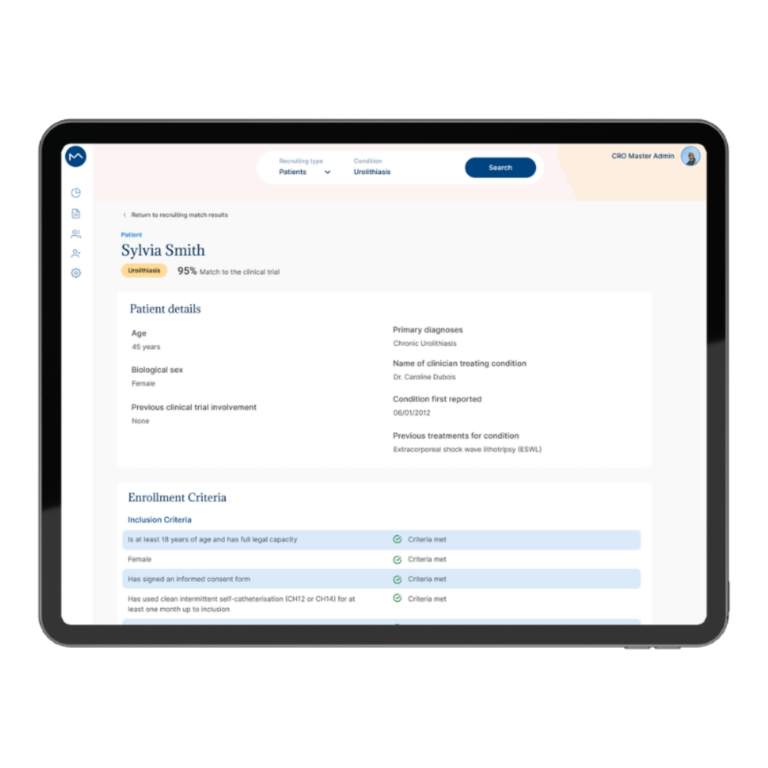
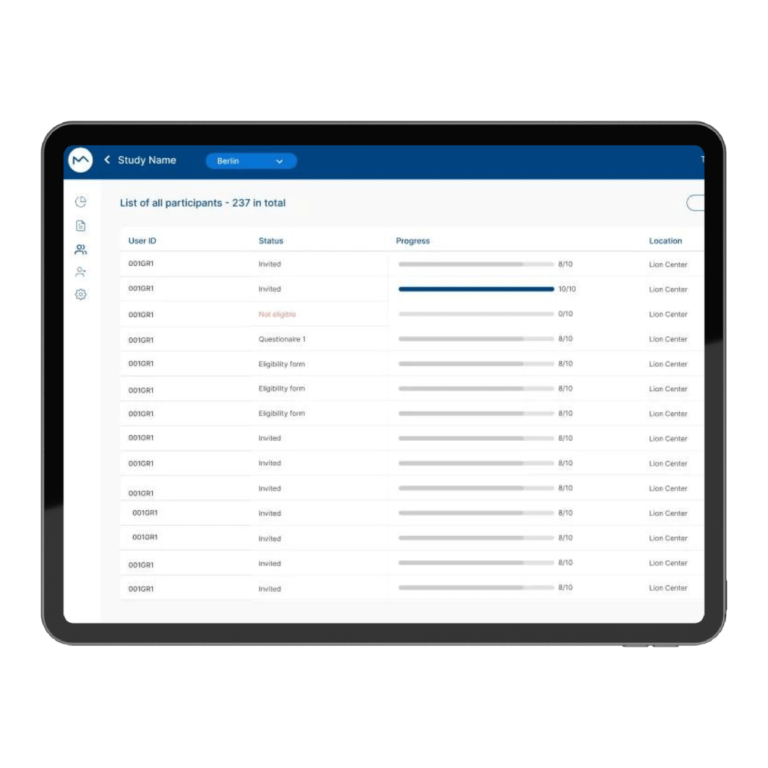
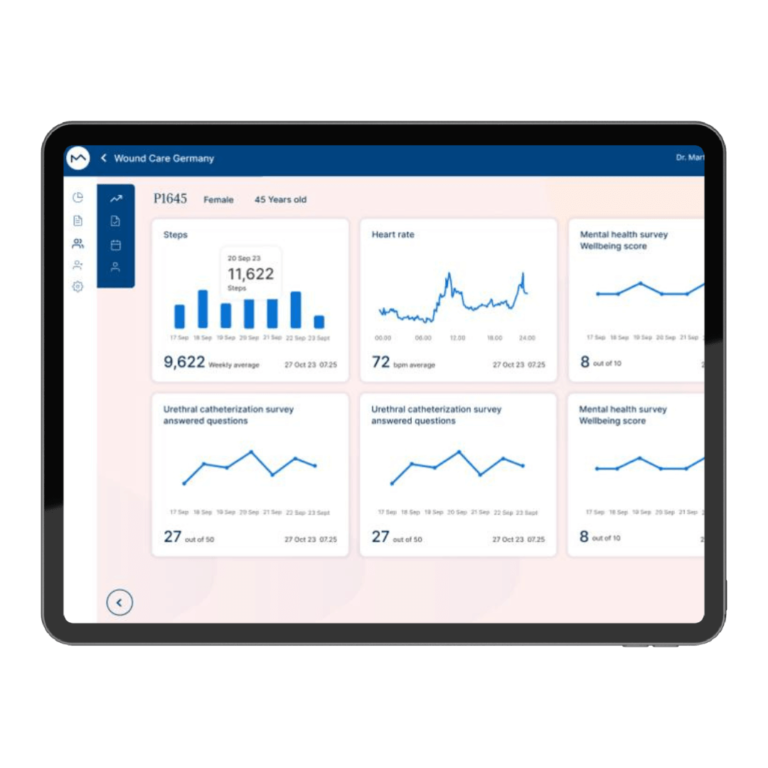
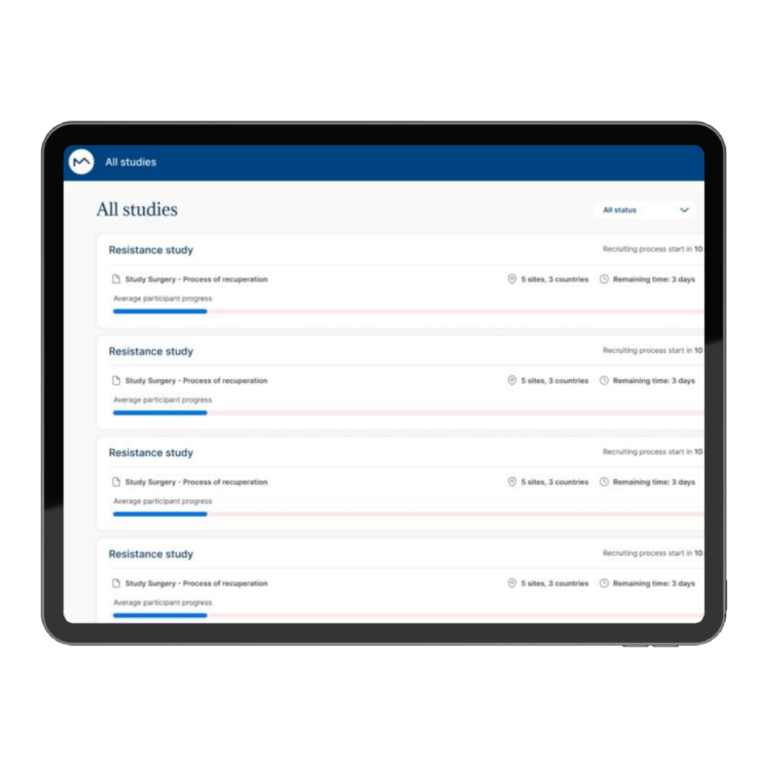
How Milo EDC Simplifies the MDD to MDR Transition
Milo EDC – Simplifying the MDD to MDR Transition Watch the Full Presentation Fill out the form below to access the complete video and gain expert insights on simplifying clinical trials with Milo eCRF. Discover the Three Principles Learn how to consolidate your systems, democratize your workflows, and integrate clinical tools from the foundation to transform your clinical trial operations. Understanding Clinical Trial Complexity The Visible Complexity Protocol design, site training, CRF setup—these are the obvious components of clinical investigations. But manufacturers often focus only on these visible elements, missing the deeper operational challenges that drive costs and timelines. The Hidden Burden Beyond protocol and site management, manufacturers face hidden operational costs and regulatory complexity. System fragmentation creates inefficiencies that compound across the entire trial lifecycle, increasing expenses and regulatory risk. The Real Cost of System Fragmentation Multiple disconnected systems requiring separate technical expertise Individual licensing costs and financial investments Duplicate data entry and workflow inefficiencies Increased complexity in regulatory compliance Longer timelines for trial setup and execution The Three Principles of Modern Clinical Trial Management 1. Consolidation Move from multiple disconnected systems to unified platforms that eliminate redundancy and reduce costs. A single platform eliminates the need for separate technical expertise and individual licensing investments. 2. Democratization No-code tools enable clinical teams to build and modify studies without IT dependencies. Empower your team to work independently, reducing timelines and improving efficiency without specialized technical knowledge. 3. Integration Embed clinical benefit tools (ePRO/eCOA) from the foundation, not as afterthoughts. Integrated tools capture real-world clinical data that strengthens regulatory submissions and demonstrates clinical benefit for MDR compliance. The Results When implemented correctly, these principles deliver faster trial setup, significantly lower operational costs, improved data quality, seamless global deployment in multiple languages, and real-world clinical data that strengthens MDR submissions. Milo EDC: Purpose-Built for the MDD to MDR Transition Milo EDC embodies all three principles with: No-Code Study Builder – Drag-and-drop functionality with live preview and embedded UAT AI-Assisted Translation – Deploy in multiple languages in seconds Integrated ePRO/eCOA – Convert CRFs to ePRO instantly with no additional cost Built-In MDR Compliance – Regulatory requirements integrated from the foundation No-Code No-Code Study Builder Drag-and-drop functionality to build EDCs without technical expertise. Live preview reduces turnaround time between development and implementation. User Acceptance Testing is embedded directly into the development cycle. AI-Assisted Translation Develop your CRF in English for EU notified body submission, then deploy in multiple languages in seconds. AI-assisted, human-controlled, expert-reviewed at every stage. Integrated ePRO/eCOA Electronic patient-reported outcomes fully integrated with no additional cost. Convert a CRF to ePRO in seconds using the same no-code builder. Captures real-world clinical data for MDR compliance. Built-In MDR Compliance Regulatory compliance is built into the foundation, not bolted on later. Every feature is designed with MDR requirements in mind, ensuring your trials meet all regulatory standards. Ready to Simplify Your Clinical Trials? Discover how Milo EDC can reduce complexity, lower costs, and improve compliance for your MDD to MDR transition. Book a Consultation