Welcome to the future of clinical trials, made more accessible through MILO DCT's integrated eConsent solution. Revolutionize the consent process by offering patients an intuitive experience, enhancing engagement, and simplifying management for clinical study teams.
MILO eConsent introduces an innovative electronic solution that complies with health authorities' regulations. It ensures that patients understand the objectives of the clinical trial and provides consent directly through the MILO DCT application.
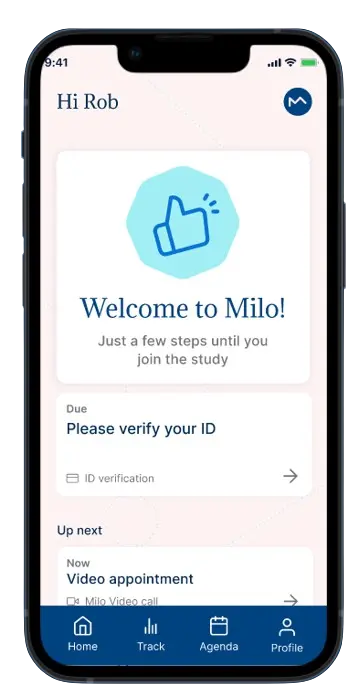
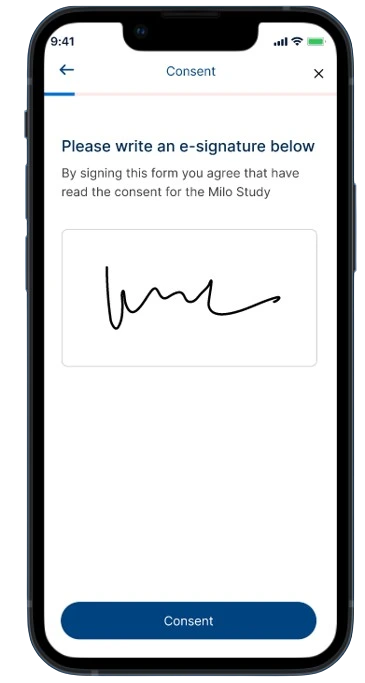
This solution streamlines the patient enrollment process, seamlessly integrating them into MILO EDC. This not only improves consent management but also reduces errors related to informed consent and lightens the administrative burden on study teams.
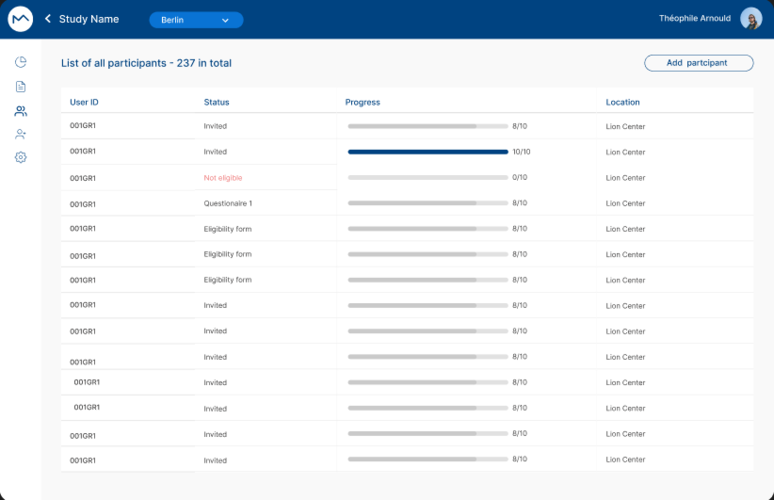
Easily track where patients are in the recruitment process after sending invitations from the MILO MATCH database. Monitor their progress and engagement effortlessly.
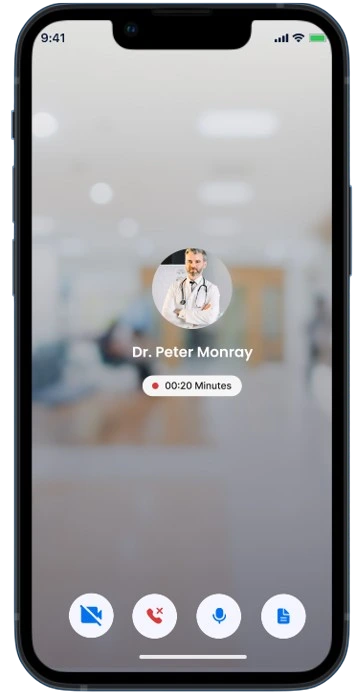
Conduct regulatory meetings with recruited patients directly through the application. Provide them with essential information, enabling them to make informed decisions regarding their participation.
Ready to enhance the accessibility of your clinical trials? Contact us now to explore the potential of MILO DCT and discover how it can simplify the consent process and improve patient engagement. Don't miss out on the opportunity to drive progress in clinical research.
Products
Industry

Company

Legal & Compliance
Newsletter