Welcome to the world of Electronic Data Capture, or EDC, a transformative solution in clinical trials that replaces traditional paper-based data collection. EDC streamlines the collection and centralization of all your research data.
Data security is paramount in clinical research, both for patients and researchers. Our EDC system is designed to ensure the confidentiality and integrity of your data. All collected information is encrypted and securely stored, compliant with stringent data protection regulations. Your research data is accessible only to the clinicians responsible for the clinical studies.
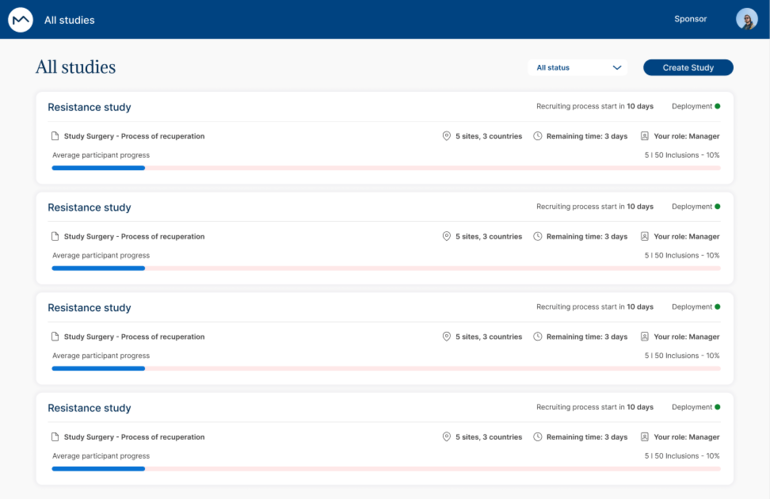
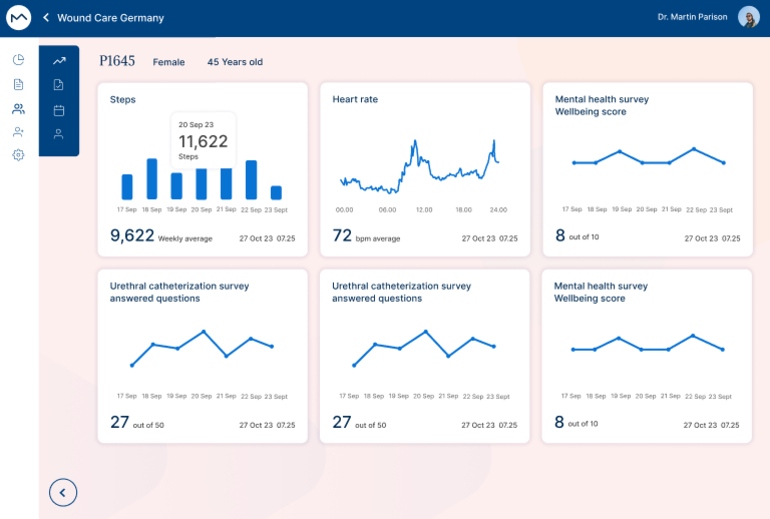
Data quality is essential for reliable research results. Our EDC simplifies the data collection process by minimizing human errors and providing an ergonomic experience for professionals. Integrated checks and automatic validations ensure high-quality data, reducing error correction costs and enhancing research accuracy.
Clinical research never stops, and our EDC is designed to keep pace. You can access your data anytime, anywhere through our online platform. No more delays due to distance or software compatibility issues. You have full control of your data, 24/7.
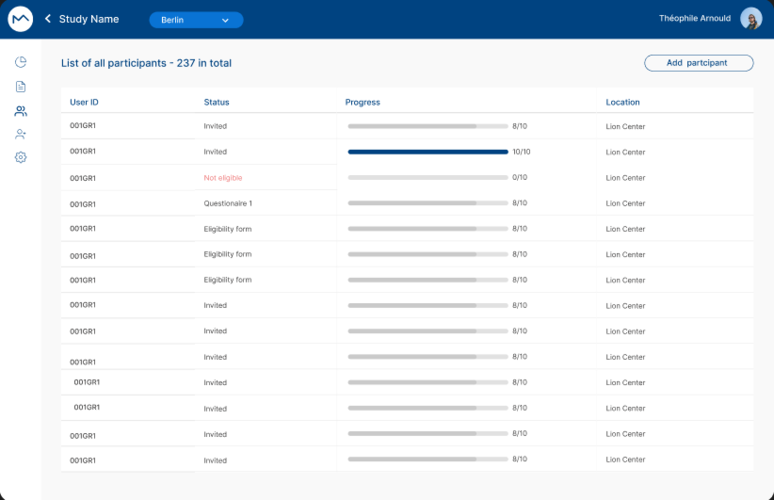
Optimize your research center management with EDC integrated into MILO DCT. Data centralization and seamless collaboration among team members simplify operations. Intuitive dashboards allow you to monitor your research's progress in real-time.
EDC isn't just a tool; it's a catalyst for research excellence. Join us in transforming the way data is collected and managed in clinical research.
Products
Industry
Company

Legal & Compliance
Newsletter