Welcome to the future of clinical data collection, where precision meets ease through MILO's Electronic Patient-Reported Outcomes, or ePro. In an era of heightened clinical trial standards, the ability to gather precise patient data is critical to success.
Direct feedback from patients is facilitated by ePRO, streamlining their participation in clinical trials. For instance, the reduced need for on-site appointments allows patients to effortlessly incorporate the upkeep of the electronic patient diary (eDiary, ePRO) into their daily routines.
This ensures direct feedback from a broader range of patients. Additionally, the straightforward handling and intuitive nature of the tools contribute to increased retention and completion rates.
Eliminate the laborious task of transcribing paper documents into Excel sheets, minimizing errors and saving significant time and resources. With digital traceability, every data alteration is documented securely.
Milo ePRO automatically provides the necessary audit trail traceability for compliance with clinical trial regulations. Mandatory fields, edit checks, input-dependent paths, and automated validations further enhance the quality of data, ensuring accuracy and reliability.
Our eCOA and ePRO solutions prioritize patient safety throughout the data collection process. Real-time data enables swift responses to adverse events, ensuring proactive intervention.
Streamline your study phases and save precious time with Milo's data analysis and visualization capabilities. Stored and pre-filtered views facilitate smoother transitions to subsequent clinical trials, accelerating future setups.
The integration of ePro within MILO DCT enables real-time patient data collection. This means you can access and analyze health data as it's entered by patients in the MILO DCT application. This guarantees informed decision-making, quicker adjustments to ongoing trials, and ultimately, greater success.
Patient engagement is pivotal to the success of any clinical study. MILO's ePro approach emphasizes user-friendly design. Patients submit their results on the MILO DCT app with simplicity and intuitiveness while ensuring the security of their data.
By minimizing the risks associated with manual data entry, MILO's ePro system enhances the accuracy and reliability of patient-reported data. As a result, the obtained results are more reliable, precisely demonstrating the value of your medical device or medication.
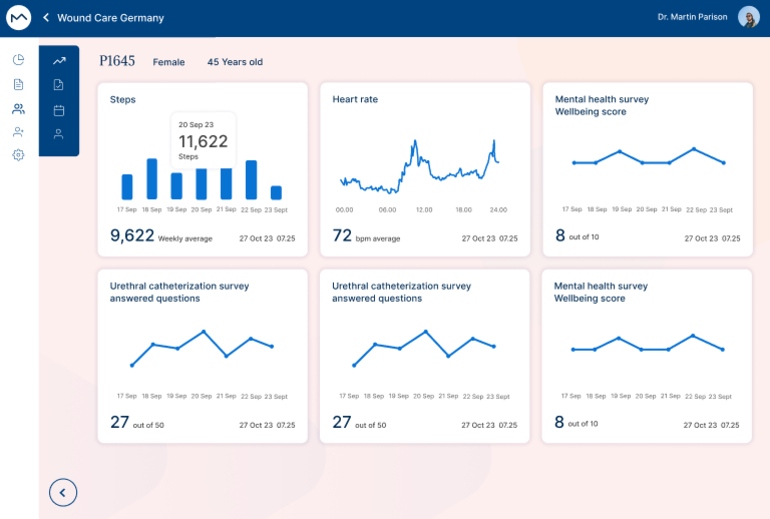
MILO ePro isn't just a tool; it's a game-changer for clinical trials. Join us in advancing the precision, reliability, and patient-friendliness of data collection in clinical research.
Products
Industry

Company

Legal & Compliance
Newsletter